Second quarter total revenue and government support income increased over 100% year-over-year
Government support income increased 52% for the quarter ended December 31, 2022 compared to the prior corresponding period, mainly due to qualifying expenditures for completion of Milestone 7
Successful completion of Milestone 7, a key phase of its biosensor platform development
Cash and cash equivalents at quarter-end totaled $2.9 million
NEW YORK, February 14, 2023 — Intelligent Bio Solutions Inc. (“Intelligent Bio Solutions” or the “Company”) (Nasdaq: INBS), a life sciences company developing and delivering intelligent, non-invasive, real-time testing solutions, today announced its financial results for its fiscal second quarter ended December 31, 2022, and provided a business update. All amounts are expressed in U.S. dollars unless indicated otherwise, and all authorized, issued, and outstanding stock and per share amounts reflect the 1-for-20 reverse stock split effected by the Company on February 9, 2023, unless indicated otherwise.
“Our strong fiscal second quarter results show the expansion and strength of our global portfolio, which now includes operations in the United Kingdom, United States and Australia,” commented Harry Simeonidis, Chief Executive Officer at Intelligent Bio Solutions. “We continued to bolster Intelligent Bio Solution’s leadership position in the field of non-invasive real-time diagnostic testing in the second quarter, achieving several significant strategic goals. We released the successful results from Milestone 7, which showed a four-time improvement in time-to-result (TTR) for our biosensor platform, returning test results in less than a minute. These exciting results were a green light for our biosensor development team to progress to the next testing phase, which will include testing human saliva.”
“Recently, we announced several customer partnerships where companies are implementing our real-time, non-invasive intelligent fingerprinting testing solutions for their staff drug tests,” continued Mr. Simeonidis. “These successful partnerships demonstrate the demand for, and effectiveness of, our innovative platform for testing needs. Based on this momentum, we believe Intelligent Bio Solutions is well positioned for future growth as we advance our development pipeline and execute our strategic priorities.”
Second Quarter Highlights, Recent Operational Developments
Strategic Partnerships & Pipeline Development
- In January 2023, the Company announced the successful completion of the review of results from Milestone 7, a phase of its biosensor platform development at the University of Newcastle, Australia. The results showed a record 4x improvement in TTR, enabling the biosensor to return test results in under one minute. The Company’s biosensor platform is the world’s first platform designed to specifically support multiple non-invasive, real-time, saliva-based diagnostic tests based on Organic Thin Film Transistor (OTFT) technology. It features a small, printable organic strip designed to put the power of accurate, timely diagnosis in the hands of patients and their primary health practitioners. The biosensor development team has now proceeded to its next testing phase, which will include testing human saliva.
- Intelligent Bio Solutions also continues to advance towards the next phase of the Biosensor development program with its second study, which focuses on eliminating the variables affecting glucose levels in saliva and in the sample collection method.
Business Development
- In February 2023, the Company announced that Boughey Distribution, one of the United Kingdom’s leading logistics providers to the food industry, has selected the on-the-spot fingerprint test from the Company’s subsidiary, Intelligent Fingerprinting (“IFP”), to support its random drug screening program, replacing its previous urine-based test.
- In January 2023, the Company announced that global garden equipment manufacturer Hozelock will implement drug tests to promote employee safety utilizing fingerprint sweat-based technology from IFP. Hozelock will use the fingerprint sweat-based drug test to determine any recent employee use of cocaine, cannabis, methamphetamine or opioids.
Commercial Development
- Intelligent Bio Solutions continues to advance the construction of its state-of-the-art manufacturing facility at the University of Newcastle in Australia specifically to produce its proprietary biosensor.
Anticipated Events and Targeted Milestones for the Coming Fiscal Year
- The launch of the Intelligent Fingerprint Drug screening solutions within the Australian and New Zealand markets, including all the required infrastructure and regulatory requirements.
- The continued development of the biosensor to test glucose, moving forward in parallel with hormone testing, focusing initially on cortisol, to be included in the Company’s next series of clinical trials anticipated to start before calendar end 2023.1. The second round of equipment for the Company’s labs, the Polymer synthesis and characterization work cells, is now complete. Intelligent Bio Solutions also completed the Installation and Equipment Qualification in November 2022.
- The advancement into the final design and verification phase of the Biosensor development with two key parallel clinical studies based on results of the recently completed blood and saliva glucose study:1. Study 1: Elimination of variables affecting glucose levels in saliva and in the sample collection method, which commenced in November 2022 and is expected to be completed in May 2023; and2. Study 2: Assessing accuracy and reproducibility of glucose testing with the Biosensor test strip, anticipated to be completed in the second half of 2023.Both studies, in tandem, are intended to refine saliva collection protocols and enhance the biosensor’s performance as the Company finalizes product design and shifts from Research and Development (“R&D”) to manufacturing.
- The determination of the scope of work for the expansion of the biosensor platform to include additional analytes, in parallel to glucose, for point of care testing using saliva.
- A focus on growing IFP product sales across the United Kingdom, mainland Europe, the Asia Pacific region and non-FDA regulated markets in the United States with significant potential.
- The submission of an FDA application for the IFP DSR system.
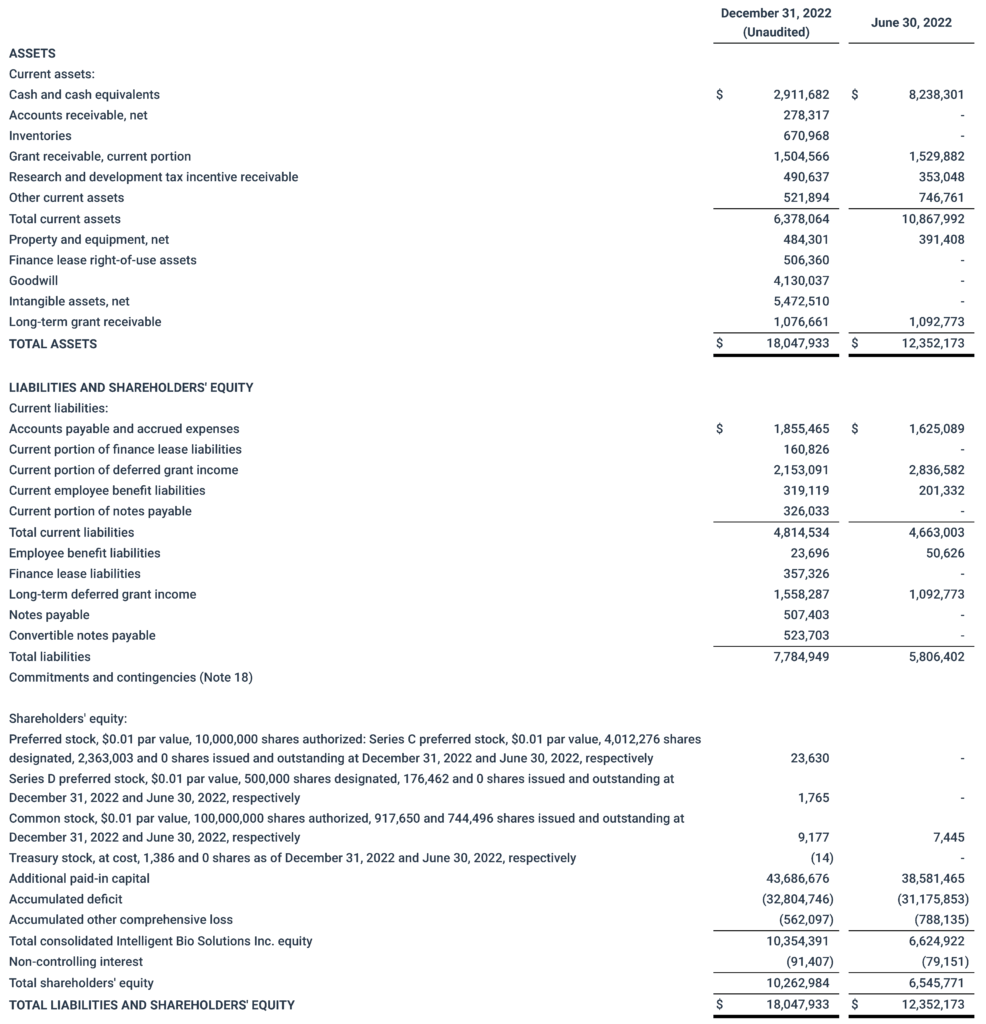
Second Quarter Ended December 31, 2022, Financial Results
As of December 31, 2022, the Company’s cash, cash equivalents and marketable securities totaled approximately $2.9 million.
For the quarter that ended December 31, 2022, the Company had a net loss of $0.42 million or $0.46 per share, compared to a net loss of $3.46 million or $4.65 per share in the prior year period. This decrease was primarily driven by expensing the prepaid R&D contribution of $2.6 million during the same period in 2021 and recognition of fair value gain on the revaluation of convertible notes and holdback Series C Preferred Stock during the current quarter of $1.8 million.
The Company’s government support income increased by 52% or $91,834 to $269,625 for the quarter ended December 31, 2022, compared to the same period in 2021. This increase was primarily attributable to the timing of the amount spent on qualifying research and development activities.
About Intelligent Bio Solutions Inc.
Intelligent Bio Solutions Inc. is a life sciences company developing and delivering intelligent, non-invasive, real-time testing solutions to customers globally. With its world-first biosensor platform, Intelligent Bio Solutions is developing and launching diagnostic tests urgently needed to help people living with chronic disease. In addition, through its recent acquisition of Intelligent Fingerprinting Limited, the Company is the world leader in the advancement of portable drugs of abuse testing through the analysis of fingerprint sweat. The system is a platform technology with potential applications in many areas of diagnostics, and its advantages include being non-invasive, hygienic, fast, and cost-effective. The top-selling product screens for recent use of the most commonly taken drugs in workplace settings; opioids, cocaine, methamphetamine, and marijuana. Sample collection takes just seconds, with results in ten minutes. Customers include employers in safety-critical industries such as construction, transport and logistics firms, drug treatment organizations, and UK coroners. A laboratory confirmation service is also available.
For more information, visit https://ibs.inc/
Forward-Looking Statements:
Some of the statements in this release are forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, Section 21E of the Securities Exchange Act of 1934 and the Private Securities Litigation Reform Act of 1995, which involve risks and uncertainties. Forward-looking statements in this press release include, without limitation, Intelligent Bio Solutions Inc.’s ability to consummate the proposed transaction described in this press release, develop and commercialize its diagnostic tests, realize commercial benefit from its partnerships and collaborations, and secure regulatory approvals, among others. Although Intelligent Bio Solutions Inc. believes that the expectations reflected in such forward-looking statements are reasonable as of the date made, expectations may prove to have been materially different from the results expressed or implied by such forward looking statements. Intelligent Bio Solutions Inc. has attempted to identify forward-looking statements by terminology, including “believes,” “estimates,” “anticipates,” “expects,” “plans,” “projects,” “intends,” “potential,” “may,” “could,” “might,” “will,” “should,” “approximately” or other words that convey uncertainty of future events or outcomes to identify these forward-looking statements. These statements are only predictions and involve known and unknown risks, uncertainties, and other factors, included in Intelligent Bio Solutions’ public filings filed with the Securities and Exchange Commission. Any forward-looking statements contained in this release speak only as of its date. Intelligent Bio Solutions undertakes no obligation to update any forward-looking statements contained in this release to reflect events or circumstances occurring after its date or to reflect the occurrence of unanticipated events.
Company Contact:
Intelligent Bio Solutions Inc.
[email protected]
Investor Contact:
Valter Pinto
KCSA Strategic Communications
[email protected]
Media Contact:
Cheryl Billson
Comma Communications
[email protected]
INTELLIGENT BIO SOLUTIONS INC.
CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE LOSS
(UNAUDITED)
(Amounts in US$)
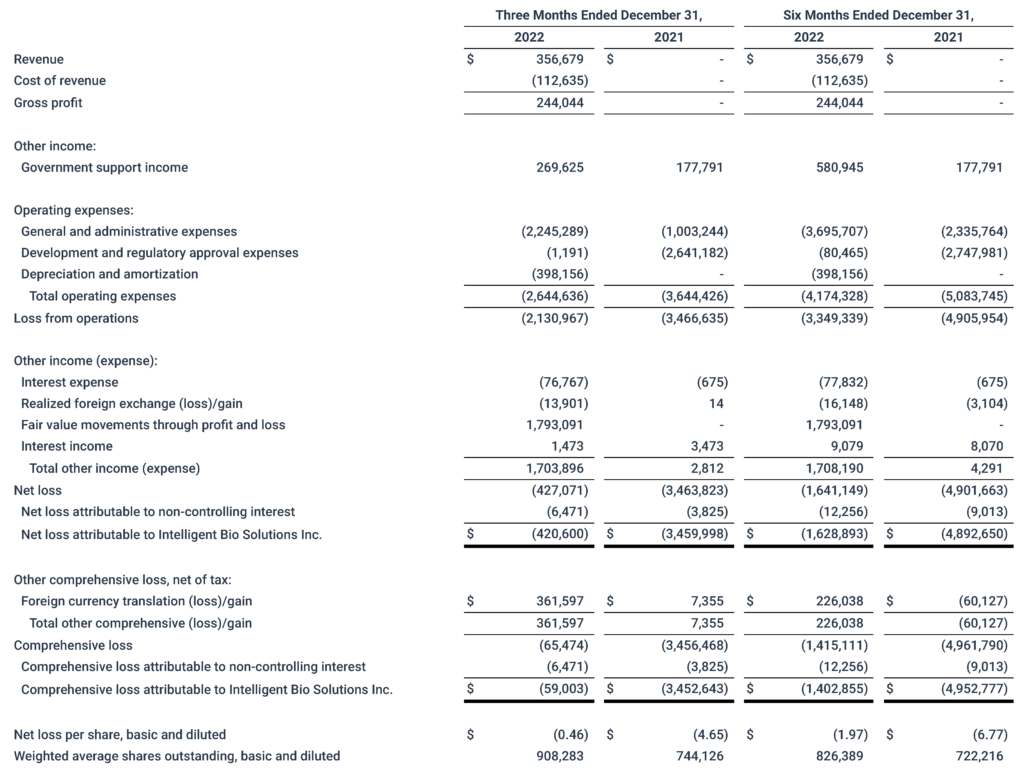
INTELLIGENT BIO SOLUTIONS INC.
CONDENSED CONSOLIDATED BALANCE SHEETS
(Amounts in US$)