Initiated prospective study to collect coincident samples of oral fluid and blood following IRB approval.
Finalizing site selection at the University of Newcastle campus for the high-tech manufacturing and production facility.
$10.76 Million in Cash, Cash Equivalents, and Marketable Securities as of March 31, 2022, provides estimated runway into H1 2023.
To host conference call and webcast today at 4:30 p.m. ET
New York, NY, May 10, 2022 – GBS Inc. (Nasdaq: GBS), a life sciences company developing non-invasive, real-time diagnostic testing in the hands of patients and their primary health practitioners at point-of-care, today announced its financial results for the third quarter ended March 31, 2022 and provided recent business updates.
“This quarter has been extremely productive for the Company as we initiated enrollment of our prospective clinical studyfollowing Institutional Review Board approval. This clinical study will provide the robust correlation data required to support future submissions/applications for key regulatory approvals in both the U.S. and Asia,”
Interim Chief Executive Officer of GBS, Dr. Steven Boyages stated.
“We have carefully designed this trial to ensure that, collectively, the subjects enrolled for clinical investigation represent a cross-section of patients living with type 2 diabetes. The clinical trial site has completed approximately one quarter of the in-clinic portion of the IRB-approved protocol and shipped the first set of samples to Johns Hopkins Hospital, Department of Pathology, where the samples will be analyzed to determine the amount of glucose in oral fluids, venous blood and capillary blood. We remain focused and committed to meeting our milestone targets and look forward to updating the marking on our progress.”
Q3 2022 Recent Developments & Status Updates
Clinical Development
Initiated Trial: Specimen Collection Methodology to Support Subsequent Correlation
Analyses of Glucose in Oral Fluids, Capillary, and Venous Blood
- The lead clinical trial site will:
- enroll approximately 40 adult subjects diagnosed with type 2 diabetes
- provide adequate distribution of age and sex
- collect and freeze saliva and blood samples for subsequent analysis
- Quest Diagnostics® and the Johns Hopkins Hospital, Department of Pathology, will perform tests on the samples to determine the amount of glucose in oral fluids, venous blood and capillary blood
- GBS will perform subsequent statistical analyses of the correlation of glucose levels
- These data will lead to the development of an algorithm between plasma and salivary glucose
Manufacturing Facility & Equipment Sourcing
Project development plan underway with objective to commence construction during September 2022
Initial sourcing for required equipment completed in April. Early equipment acquisition allows for a more efficient build out process while at the same time ensuring the onsite manufacturing equipment is set in preparation for regulatory inspection of the facility.
Delivery of the initial batch of equipment is expected June 2022
Key Clinical & Operational Milestone Targets
- Initial data from time-course correlation study by July 2022
- Preliminary Biosensor response by September 2022
- Site selection completed with University of Newcastle by August 2022
- Delivery of required equipment for manufacturing build out by December 2022
- Biosensor Algorithm Development by December 2022
Third Quarter 2022 Financial Results
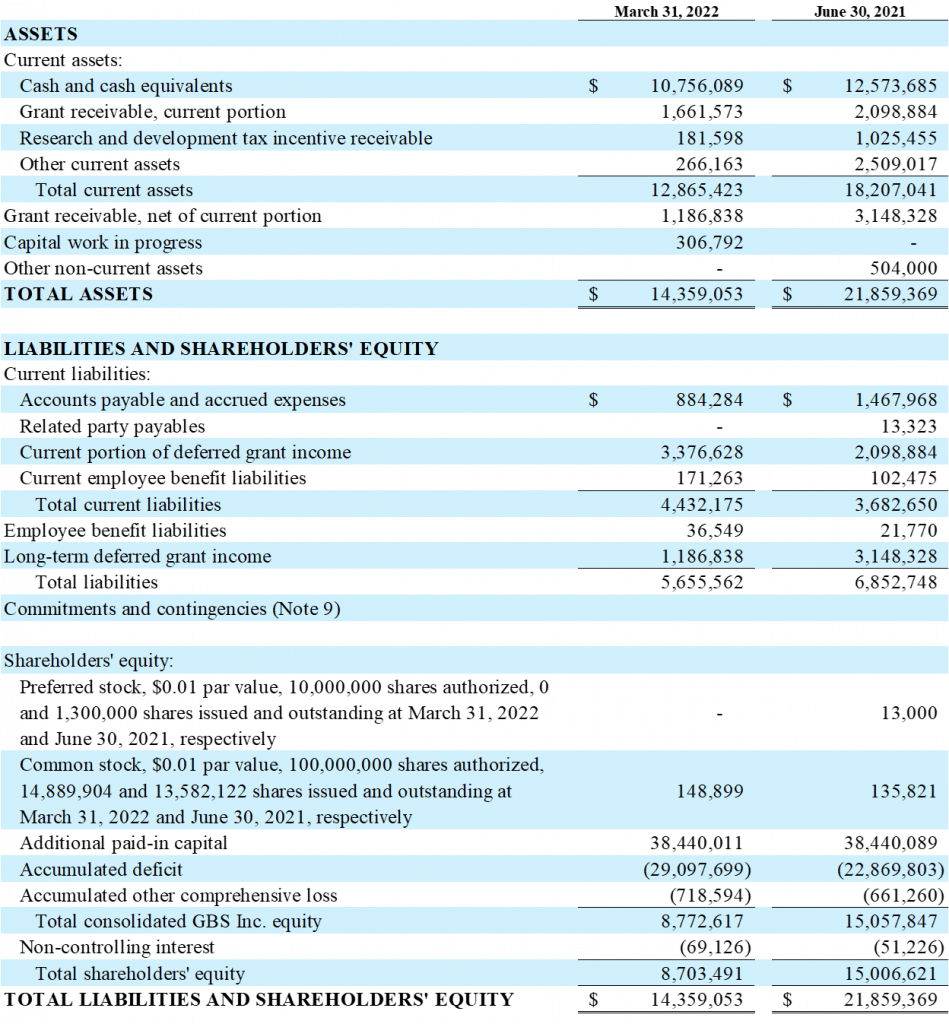
As of March 31, 2022, the Company’s cash, cash equivalents and marketable securities totaled approximately $10.76 million, compared to approximately $12.57 million on June 30, 2021.
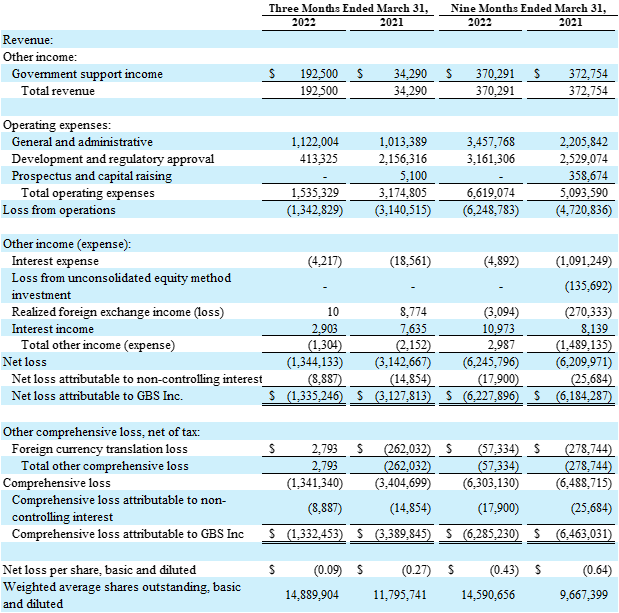
For the quarter ended March 31, 2022, the Company had a preliminary net loss of $1.34 million or $0.09 per share, compared to a net loss of $3.14 million or $0.27 per share for the same period in 2021. This is predominantly due to the timing of R&D expenditures and government support income.
As of March 31, 2022, the Company had 14.90 million shares outstanding.
Based on the current operating plan and financial resources, management believes its cash, cash equivalents and marketable securities at March 31, 2021, are sufficient to cover expenses and capital requirements into H1 2023. Our cash runway projection does not include exercising the glucose North American license option agreement.
Conference Call and Webcast
To participate in today’s conference call, please dial 855-327-6837 (Domestic/Toll-Free) or
631-891-4304 (International) and reference the conference ID: 10018968.
To participate via a webcast, please visit: Webcast Registration Link
The webcast will be archived for approximately 30 days and will be available at https://investors.gbs.inc/news-and-events/investor-calendar
About GBS Inc.
GBS Inc. is a life sciences company developing non-invasive, real-time monitoring and diagnostic tests for patients and their primary health practitioners. With the world-first Biosensor Platform, GBS Inc. is developing and launching diagnostic tests urgently needed to help people living with diabetes. For more information, please visit GBS.inc or follow GBS Inc. on Twitter and LinkedIn
Forward-Looking Statements:
Some of the statements in this release are forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, Section 21E of the Securities Exchange Act of 1934 and the Private Securities Litigation Reform Act of 1995, which involve risks and uncertainties. Forward-looking statements in this press release include, without limitation, GBS Inc.’s ability to develop and commercialize its diagnostic tests, realize commercial benefit from its partnerships and collaborations, and secure regulatory approvals, among others. Although GBS, Inc. believes that the expectations reflected in such forward-looking statements are reasonable as of the date made, expectations may prove to have been materially different from the results expressed or implied by such forward-looking statements. GBS Inc. has attempted to identify forward-looking statements by terminology including ”believes,” ”estimates,” ”anticipates,” ”expects,” ”plans,” ”projects,” ”intends,” ”potential,” ”may,” ”could,” ”might,” ”will,” ”should,” ”approximately” or other words that convey uncertainty of future events or outcomes to identify these forward-looking statements. These statements are only predictions and involve known and unknown risks, uncertainties, and other factors, included in the Company’s public filings filed with the Securities and Exchange Commission. Any forward-looking statements contained in this release speak only as of its date. We undertake no obligation to update any forward-looking statements contained in this release to reflect events or circumstances occurring after its date or to reflect the occurrence of unanticipated events.
For more information, please contact:
Alex Arzeno – Vice President of IR & Communications, GBS, Inc.
[email protected]
Investor Contact:
Tim McCarthy – Managing Director, LifeSci Advisors, LLC
[email protected]
GBS, INC. PRELIMINARY CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE LOSS (UNAUDITED)
(Amounts in $)
GBS, INC.
PRELIMINARY CONSOLIDATED BALANCE SHEETS
(Unaudited)
(Amounts in $)